Item NO.:
NRM-FT- 142(152)-1/2Payment:
T/TSpecification:
1/25 Tests
Novel Coronavirus (2019-nCoV) Antigen Testing Kit (Colloidal Gold)
Specification: 25 Tests
No professional testing equiment is required
Give the result within 20 minutes with high accuracy
Store at room temperature and transport at normal temperature Valid for two years
It is easy to operate without professional guidance
Product Quality
Passed the China Food and Drug Inspection and Testing Institute
Passed the performance test of two independent laboratories in the United States
2019-nCoV rapid test for antigen Advantages VS PCR kit
|
Test |
Equipment |
Test Time |
Transporation&Storage |
Validity |
Operation |
|
Antigen kit |
unnecessary |
20 minutes |
Room Temperature |
24months |
Easy |
|
PCR kit |
unnecessary |
2-3hours |
Cold-chain transportation& Storage |
12months |
Complex |
Comparing PCR,Esaier operation and less limits in storage&delivery
Fast results, excluding site and instrument limits.
Facilitate rapid screening on a large scale and reduce infectious possibility during patient queuing period
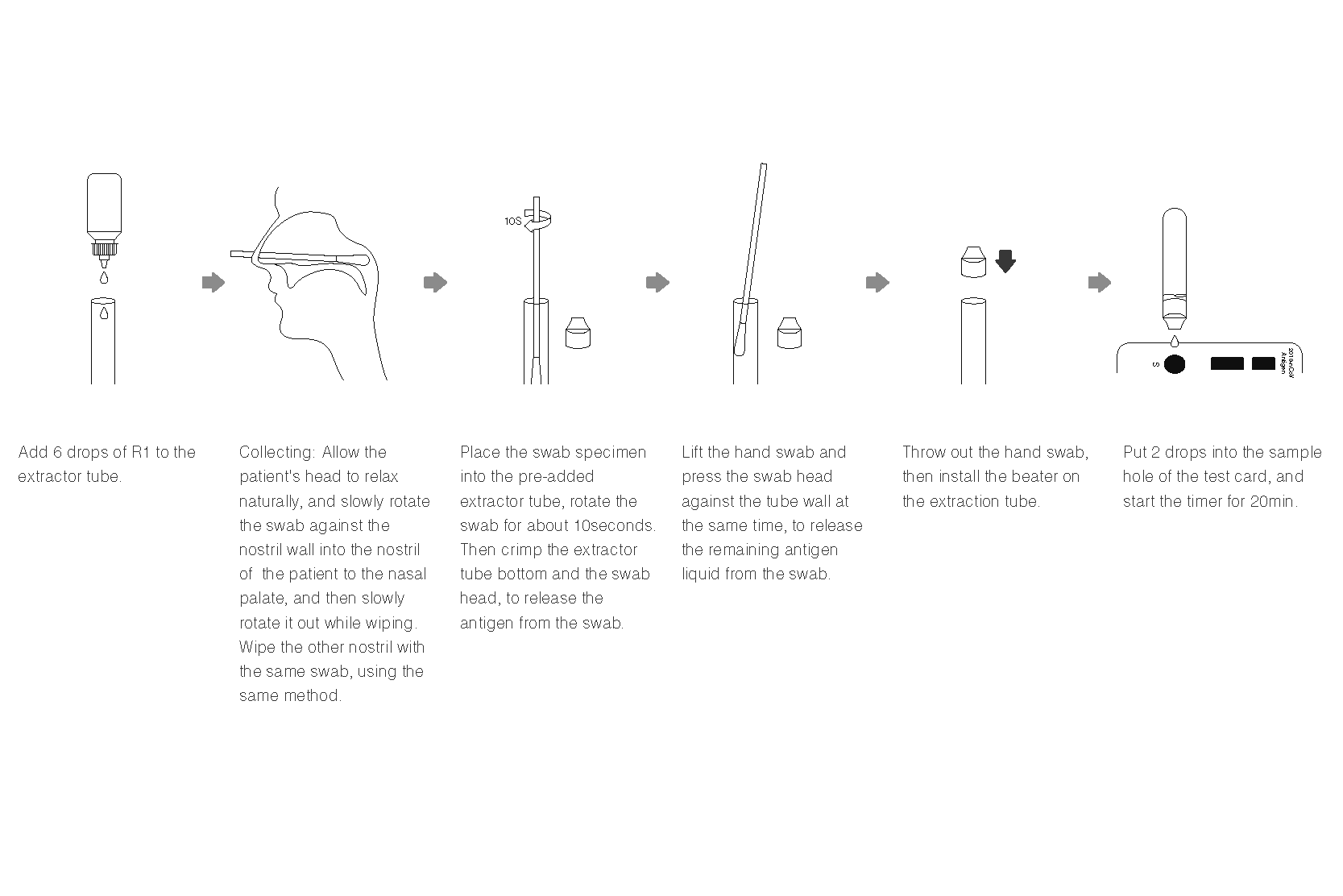
Instruction For Use (Nasopharyngeal Swab)
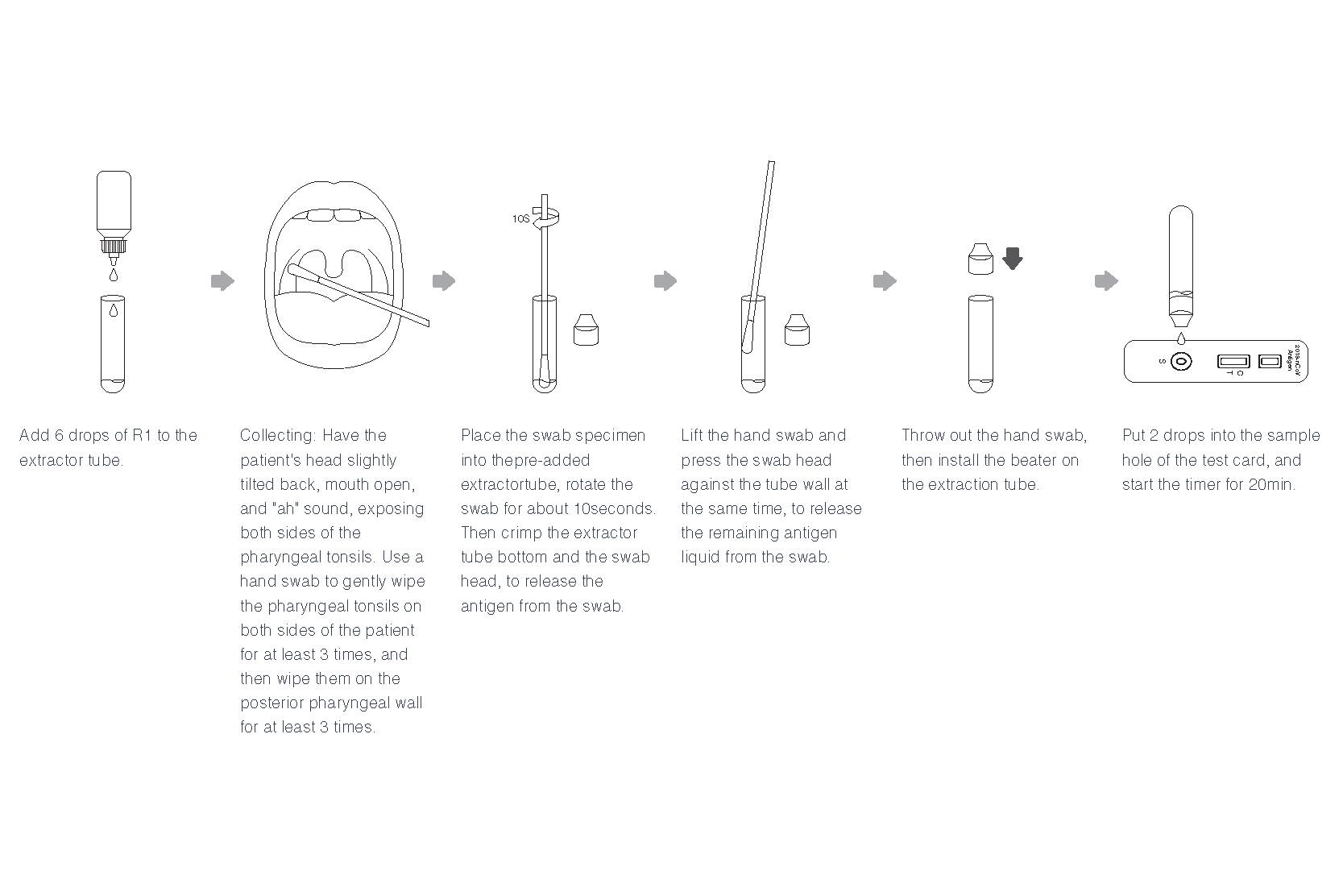
 Instruction For Use(Oropharyngeal Swab)
Instruction For Use(Oropharyngeal Swab)
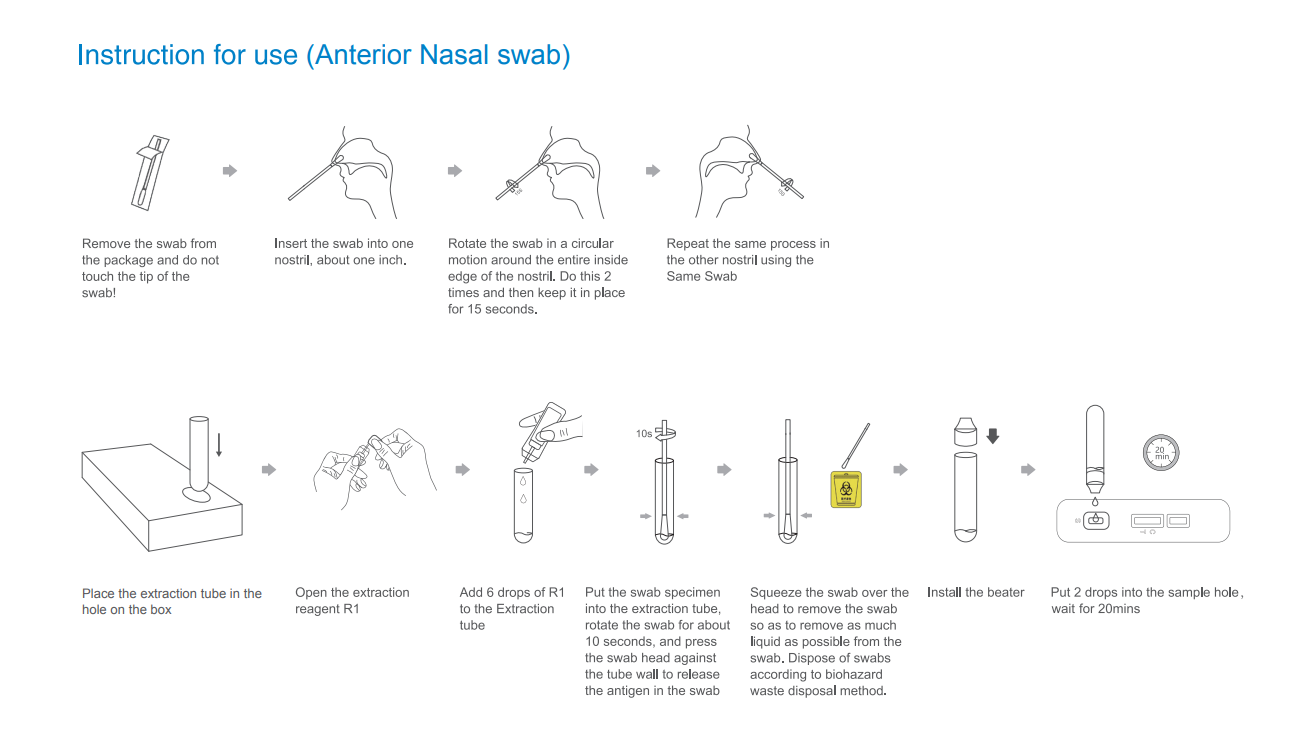
Instruction For Use(Anterior Nasal Swab)
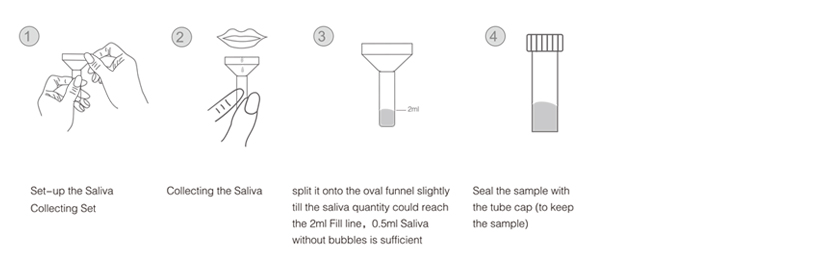
Instruction For use(saliva)
Specimen Collection
Test Procedures
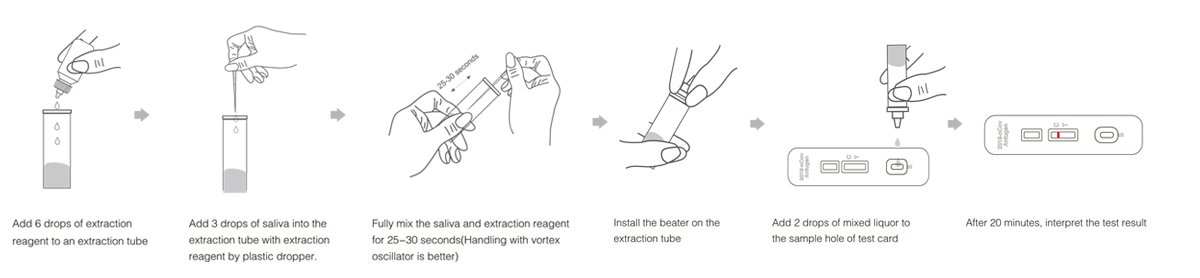
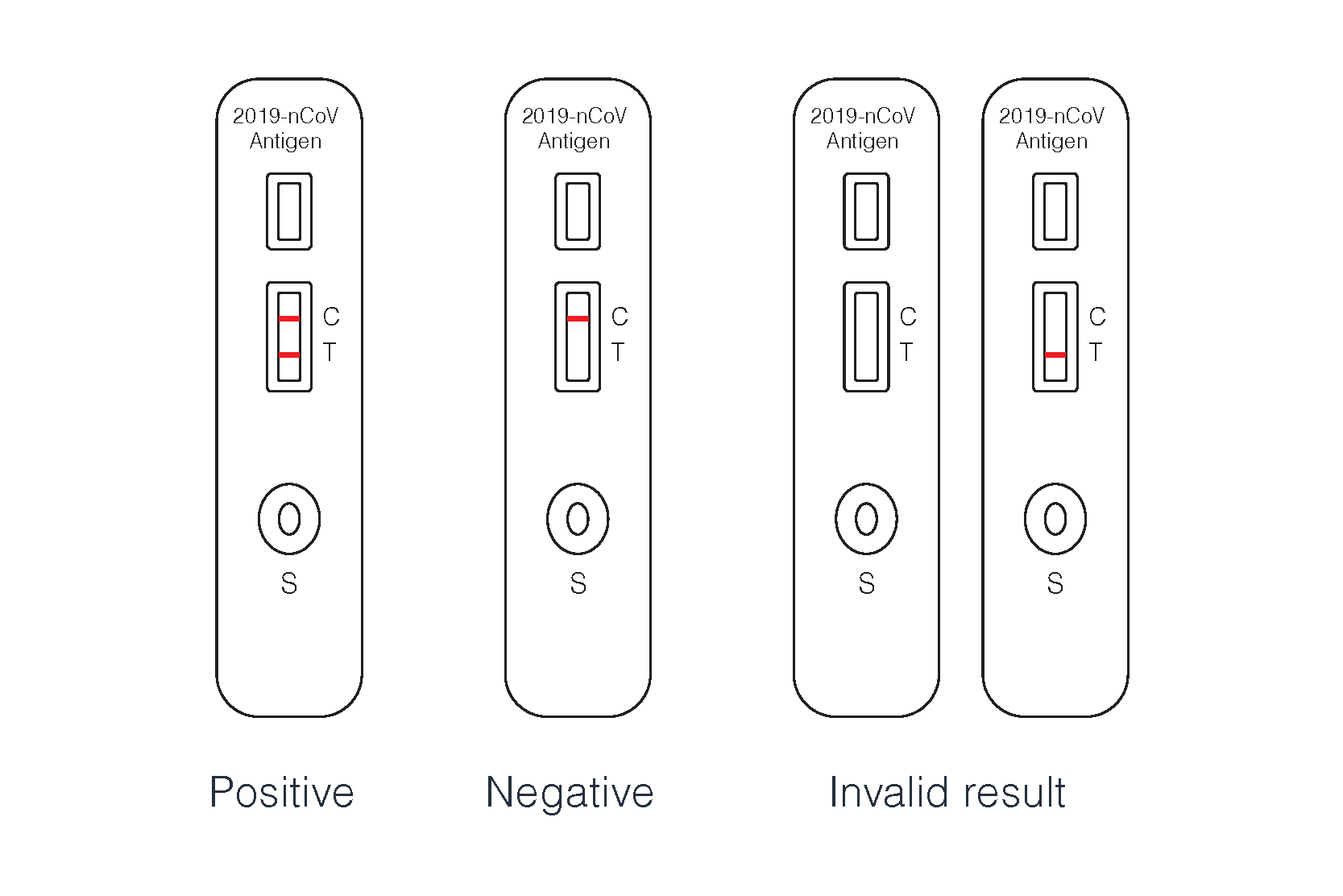
Result analysis
For the result after 30 minutes is invalid,so negative result must be given after 20 minutes, A strong positive result must be given within 20 minutes, and do final review in 20 minutes.
Applicable Place
Screening of 2019-nCoV patients for convenience of health professional
Rapid diagnosis of asymptomatic personnel;
Monitoring public health, assessing local 2019-nCoV infection rate and predicting disease development.