 English
English
 English
English
From February 10 to 13, 2026, World Health Expo Labs Dubai (formerly Medlab Middle East) was grandly held at the Dubai World Trade Centre. As a key exhibition in the field of laboratory medicine and in vitro diagnostics across the Middle East with global reach, this year’s event brought together more than 850 companies from over 50 countries and regions and attracted professional visitors from more than 180 countries and regions.
Nanjing Norman Biological Technology Co., Ltd. (hereinafter referred to as “Nanjing Norman”) participated in the exhibition with the NORMAN-CL211 series benchtop rapid chemiluminescence platforms, the NRM 411 series high-throughput chemiluminescence platforms, the FI series POCT platforms, and approximately 200 testing reagents.
Exhibition Highlights and Product Showcase
At this exhibition, Nanjing Norman primarily presented the following products and solutions:
NORMAN-CL211 Series Benchtop Rapid Chemiluminescence Immunodiagnostic Platform:
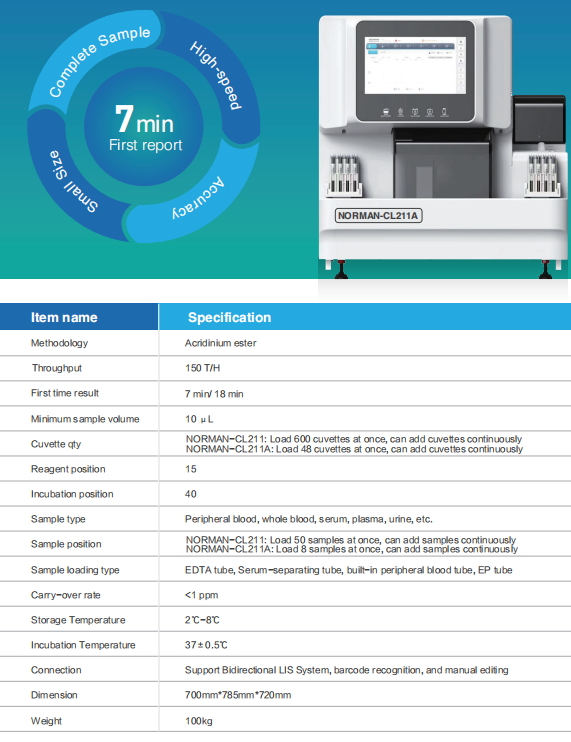
In outpatient and emergency testing scenarios, “speed” and “accuracy” have always been the most critical clinical requirements. However, existing technologies often struggle to balance both: POCT products provide rapid results but have relatively limited accuracy and sensitivity, while traditional chemiluminescence platforms offer high accuracy yet are constrained by testing workflows and reaction times, making it difficult to meet the time-sensitive demands of emergency settings.The NORMAN-CL211 series benchtop rapid chemiluminescence immunodiagnostic platform exhibited by Nanjing Norman integrates miniaturized design, rapid detection capability, and high sensitivity, achieving a balance between “speed” and “accuracy.” The platform supports both STAT (emergency) and routine dual testing modes:
In STAT mode, the reaction time between samples and reagents is shortened to 7 minutes, enabling rapid detection of key indicators such as inflammation, myocardial injury, heart failure, and acute kidney injury.
In emergency situations, it supports direct whole-blood testing without centrifugation, significantly reducing the overall turnaround time from sampling to reporting, truly achieving “collect-and-test immediately, test-and-report immediately.”
For infants and populations with difficult blood collection, Nanjing Norman has introduced a peripheral micro-volume testing solution, requiring only 10–20 microliters of samples to complete multiple inflammation and infection tests, reducing repeated blood draws and improving patient comfort and compliance, highlighting a human-centered design oriented toward clinical needs.
The platform is particularly suitable for outpatient clinics, emergency departments, chest pain centers, pediatrics, and other clinical scenarios with extremely high requirements for testing timeliness, helping physicians gain valuable golden treatment time while ensuring reliable and accurate results.
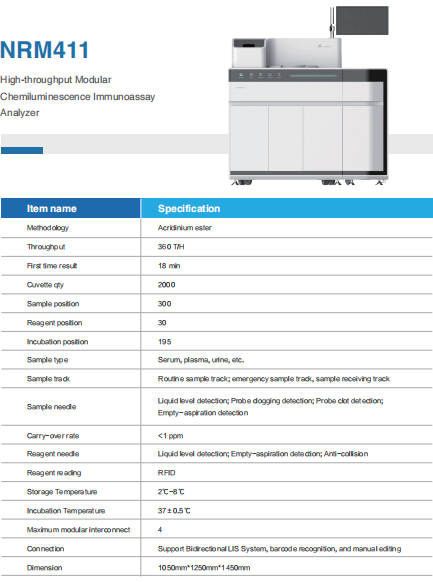
NRM 411 Series High-Throughput Chemiluminescence Platform: Designed for large general hospitals and third-party laboratories, covering more routine and specialized testing needs.
FI Series POCT Platform: Adapted for primary healthcare institutions and small testing sites, providing convenient and rapid on-site testing capability.

Reagent Portfolio
Norman’s chemiluminescence platforms cover more than 140 immunoassay reagents across 16 major clinical fields, including oncology, inflammation, cardiovascular diseases, thyroid function, hormones, anemia, autoimmune diseases, diabetes, bone metabolism, liver fibrosis, renal function, neurological injury, therapeutic drug monitoring, thrombosis, and infectious diseases.
The FI series POCT platform covers approximately 50 commonly used testing items, including thyroid function, inflammation, bone metabolism, oncology, hormones, autoimmune diseases, diabetes, cardiovascular diseases, and renal function.
At this exhibition, we grandly launched two patented products: the soluble suppression of tumorigenicity 2 protein test kit (sST2, Patent No.: ZL 2025 1 0347979.8) and the heparin-binding protein test kit (HBP, Patent No.: ZL 2025 1 0852702.0).
sST2 is a plasma biomarker associated with myocardial stress/inflammation and fibrosis. It is used for risk stratification and prognostic evaluation in heart failure. Elevated levels are significantly correlated with mortality and heart-failure readmission rates. It complements traditional indicators (such as BNP/NT-proBNP) and provides additional information in predicting long-term outcomes and treatment responses, particularly demonstrating greater robustness in patients with renal insufficiency or certain clinical conditions.
HBP is an inflammatory mediator released by neutrophils and rises rapidly in the early stages of systemic infection/sepsis. It can serve as an early diagnostic and warning indicator to identify high-risk patients likely to progress to organ failure or shock. It complements existing inflammatory/infection markers (such as PCT and CRP). In several studies and meta-analyses, HBP has demonstrated good diagnostic and prognostic discrimination for severe infections/sepsis, improving early identification rates.
On-Site Response and International Cooperation Opportunities
During the exhibition, Nanjing Norman’s booth attracted a large number of professional visitors, distributors, and medical institution representatives from the Middle East, Africa, Southeast Asia, South Asia, Central Asia, and Europe. The company’s team conducted in-depth discussions and matchmaking with multiple partners regarding localized registration, channel development, product promotion, and long-term cooperation models. Customers generally gave high recognition and positive evaluations of the rapid performance, stability, and scenario adaptability of the company’s chemiluminescence platforms.
Corporate Philosophy and Future Outlook
Nanjing Norman has always adhered to a product development philosophy guided by clinical needs, emphasizing the integration of technological innovation with user experience. In the future, the company will continue to deepen its focus on the immunodiagnostics field, further enrich its product portfolio and testing menu, accelerate international expansion, strengthen localized services and channel development, and strive to provide efficient, accurate, and reliable testing solutions for more medical institutions worldwide, integrating technological achievements into every individual’s life.